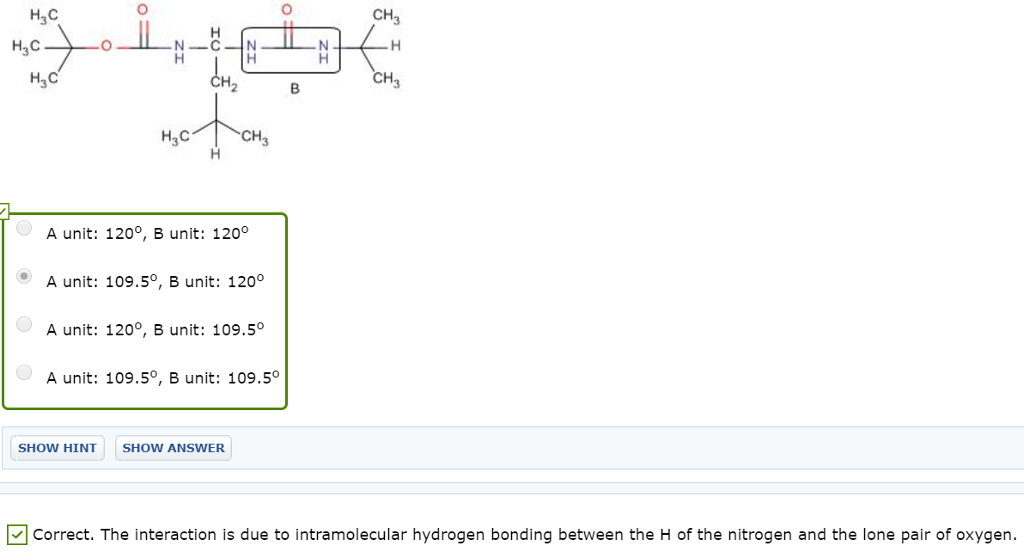
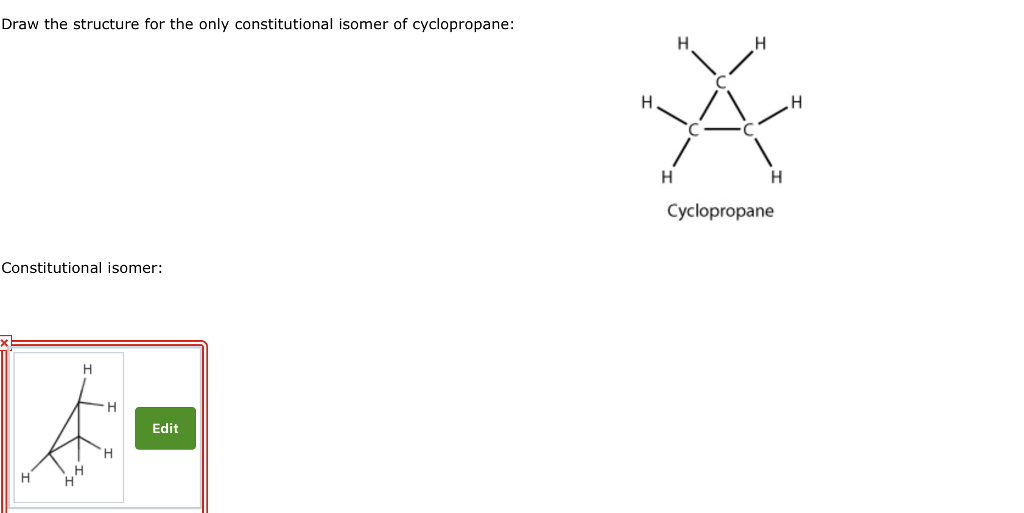
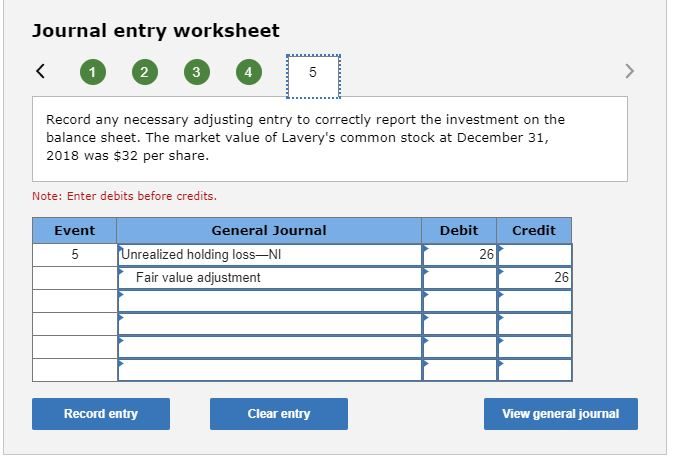
On January 4, 2018, Runyan Bakery paid $346 million for 10 million shares of Lavery Labeling Company common stock. The investment represents a 30% interest in the net assets of Lavery and gave Runyan the ability to exercise significant influence over Lavery’s operations. Runyan chose the fair value option to account for this investment. Runyan received dividends of $3 per share on December 15, 2018, and Lavery reported net income of $260 million for the year ended December 31, 2018. The market value of Lavery’s common stock at December 31, 2018, was $32 per share. On the purchase date, the book value of Lavery’s net assets was $910 million and:
The fair value of Lavery’s depreciable assets, with an average remaining useful life of [a(27)] years, exceeded their book value by $60 million.
The remainder of the excess of the cost of the investment over the book value of net assets purchased was attributable to goodwill.
Required:
1-a. Prepare all appropriate journal entries related to the investment during 2018, assuming Runyan accounts for this investment under the fair value option, and accounts for the Lavery investment in a manner similar to what it would use for securities for which there is not significant influence.
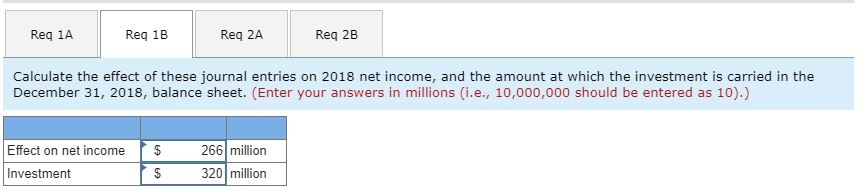
1-b. Calculate the effect of these journal entries on 2018 net income, and the amount at which the investment is carried in the December 31, 2018, balance sheet.
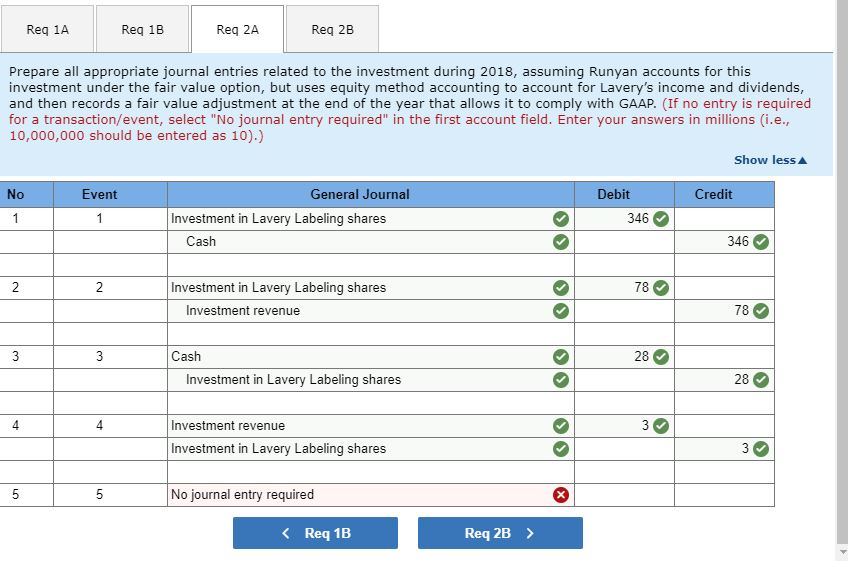
2-a. Prepare all appropriate journal entries related to the investment during 2018, assuming Runyan accounts for this investment under the fair value option, but uses equity method accounting to account for Lavery’s income and dividends, and then records a fair value adjustment at the end of the year that allows it to comply with GAAP.
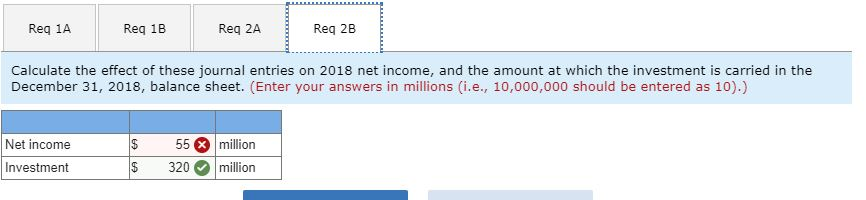
2-b. Calculate the effect of these journal entries on 2018 net income, and the amount at which the investment is carried in the December 31, 2018, balance sheet.
Req. 1A
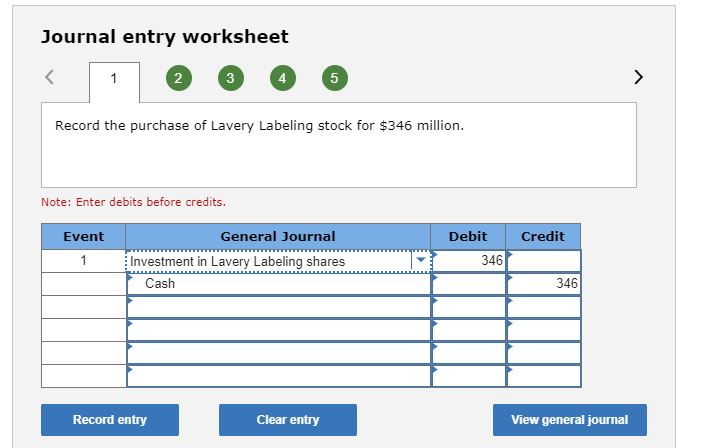
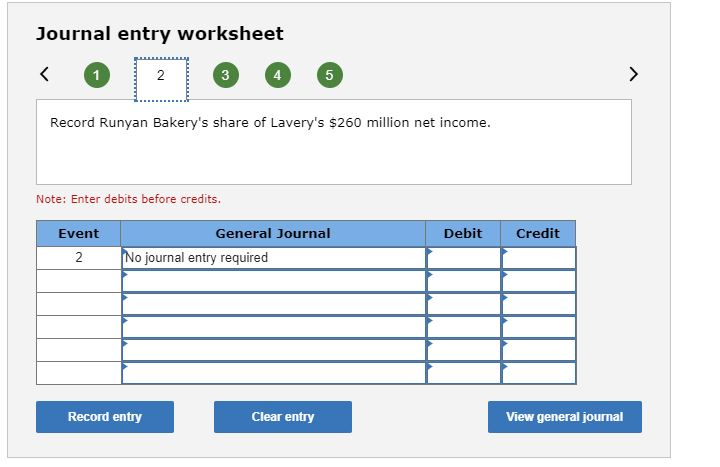
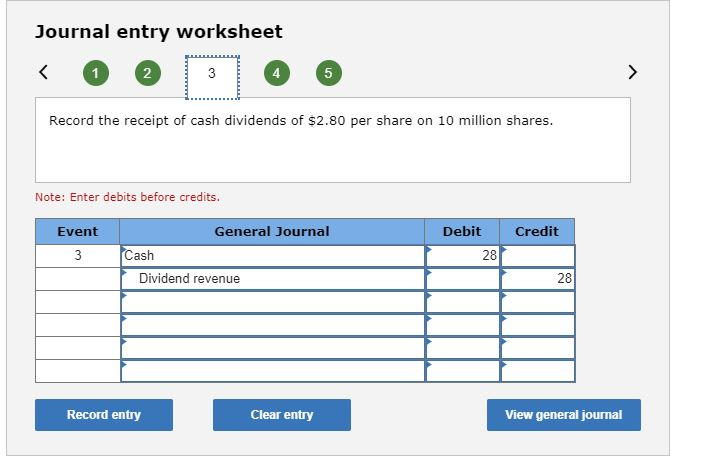
I think I made a couple of mistakes on the problem, but I’m not sure what is wrong specifically.Journal entry worksheet 2 3 4 5 Record the purchase of Lavery Labeling stock for $346 million. Note: Enter debits before credits. Event General Journal Debit Credit Investment in Lavery Labeling shares 346 Cash 346 Record entry Clear entry View general journal
![e Microsoft Edge 园Start Chapter 3 Hw × □ students ezto.mheducation.com/hm.tpx M3-13 Preparing Accrual Basis Journal Entries for Business Activities [LO 3-3] Quick Cleaners, Inc. (QCI) has been in business for several years. It specializes in cleaning houses but has some small business clients as well. a. Issued $27,000 of QCI stock for cash b. Incurred $890 of utilities costs this month and will pay them next month. c. Paid wages for the current month, totaling $1,850 d. Performed cleaning services on account worth $3,200 e. Some of Quick Cleaners equipment was repaired at a total cost of $254. The company paid the full amount at the time the repair work was done. 1. Prepare journal entries for the above transactions, which occurred during a recent month (If no entry is required for a transaction/event, select No Journal Entry Required in the first account field.) View transaction list Journal entry worksheet Issued $27,000 of QCI stock for cash. Record the transaction. Note: Enter debits before credits General Journal Record entry Clear entry View general journal](https://media.cheggcdn.com/media%2F547%2F5477a2bb-3f15-4e94-8655-5e99682478b5%2Fimage)
![ACC2013-04 3 Hw Question 4 (of 26) value 0.42 points M3-10 Identifying Accrual Basis Expenses [L。32] The following transactions are February activities of Swing Hard Incorporated, which offers indoor golfing lessons in the northeastern United States. If an expense is to be recognized in February, indicate the amount. (Enter zero for any transactions that have a net effect of zero on the income statement.) Activity Swing Hard paid $5,600 to its golf instructors for the month of February Swing Hard paid $2.650 for electricity used in the month of January b Swing Hard received an electricity bill for $1.,640 for the month of February, to be paid in C March References eBook & Resources Worksheet Dimculity 1 Easy M3-10 Identifying Acerual Basis Expenses [Lo 3-21 Leaming Objective: 03-02 Explain and apply the revenue and expense](https://media.cheggcdn.com/media%2Fb67%2Fb67821fa-811c-4473-812f-fb261a8f261b%2Fimage)