
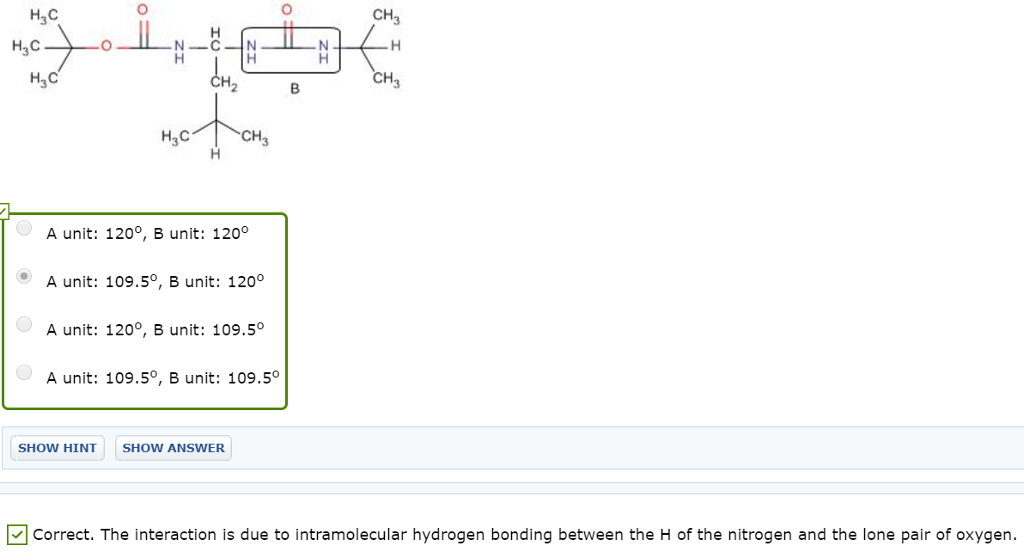
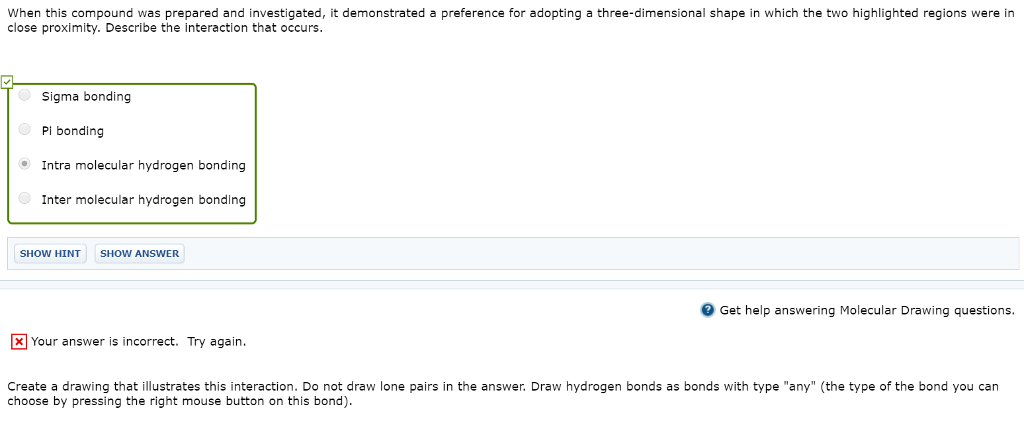
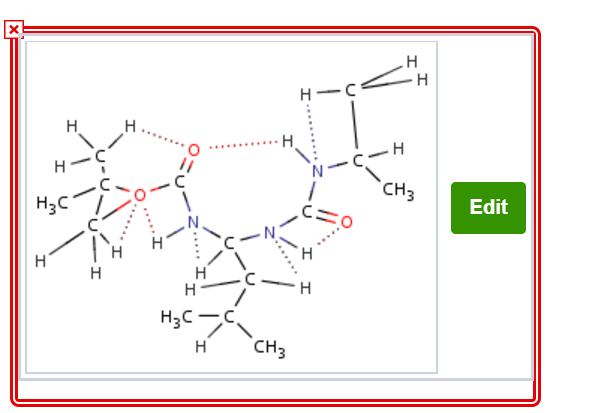
The study of analogues of small peptide chains, called peptidomimetics, provides models to understand the observed stabilizing effects in larger peptides, including enzymes (nature’s catalysts). The following structure shows promise for studying how enzymes coil up into very discrete shapes that endow them with catalytic function (Org. Lett. 2001, 3, 3843-3846): H CH2 H H CH Correct. The A unit carbon atom is sp3 hybridized expected to be tetrahedral geometry and the B unit carbon atom is sp2 hybridized expected to be trigonal planar geometry This compound has two N-C-N units, with differing bond angles. Predict the difference in bond angles between these two units H3C H3C CH CH
Expert Answer
A molecule central atom involve in Sp^3 hybridization shape is tetrahydral and bond angle is 109.50
A unit bond angle is 109.50
A molecule central atom involve in Sp^2 hybridization shape is planar triangle and bond angle is 1200
B unit bond angle is 1200
A unit: 109.50 , B unit: 1200
2.
intermolecular hydrogen bond >>>>answer
N-H………O

