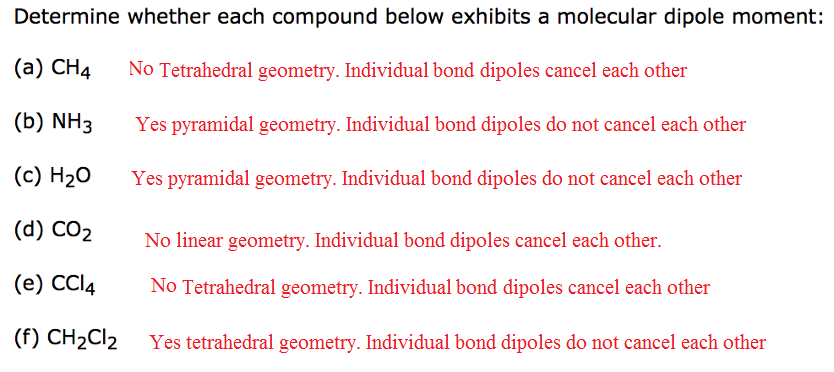
Determine whether each compound below exhibits a molecular dipole moment: (a) CH_4 (b) NH_3 (c) H_2 O (d) CO_2 (e) CCl_4 (f) CH_2 Cl_2
Expert Answer
Answer
If a molecule is symmetric, individual bond dipoles cancel each other and the molecular dipole moment is zero.
If a molecule is non symmetric, individual bond dipoles do not cancel each other and the molecular dipole moment is non zero.