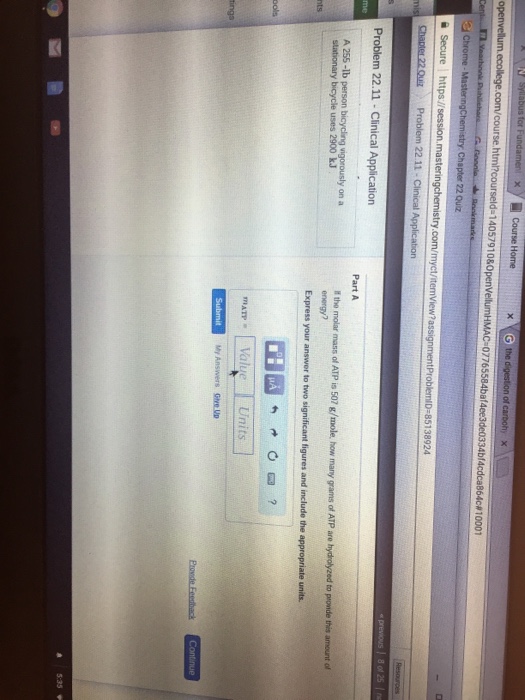
Of c 14057910&0 22.11 Clinical energy?
Expert Answer
Answer
Energy released by hydrolysis of 1 mole of ATP = -57 kJ/mol
So for 1700 kJ we would need = 2900 kJ/57 kJ/mol = 50.877 mol ATP
grams of ATP hydrolyzed = 50.877 mol x 507 g/mol = 25794.737 g
please rate.

