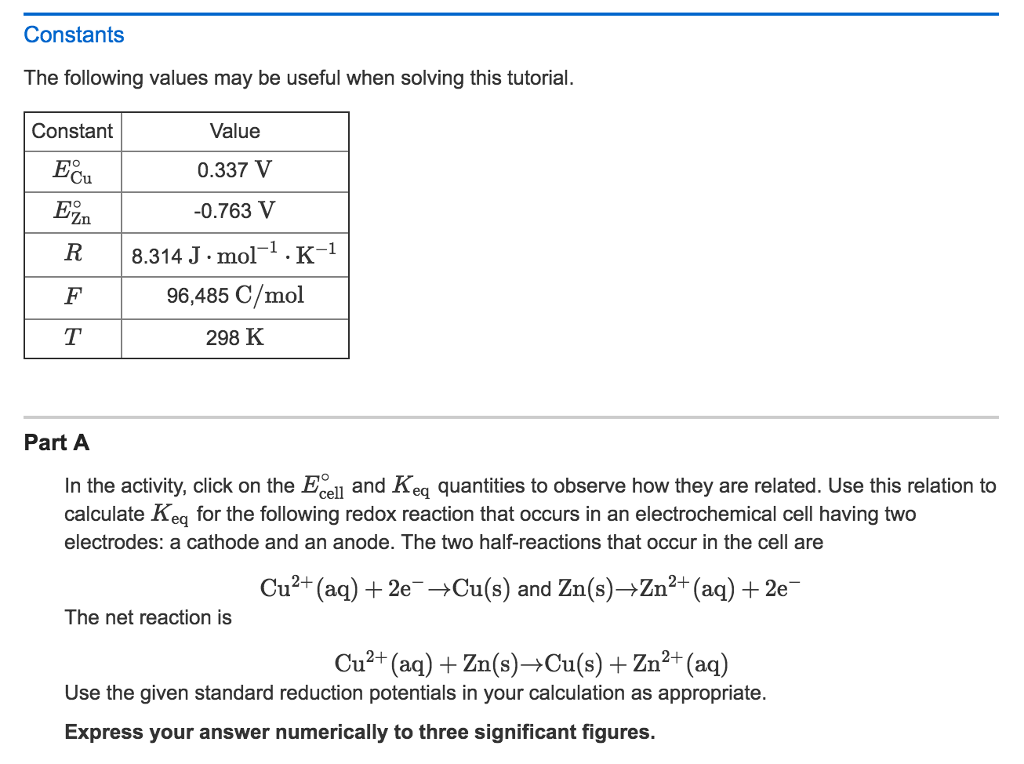
Constants The following values may be useful when solving this tutorial. Part A In the activity, click on the E degree _cell and K_eq quantities to observe how they are related. Use this relation to calculate K_eq for the following redox reaction that occurs in an electrochemical cell having two electrodes: a cathode and an anode. The two half-reactions that occur in the cell are Cu^2+ (aq) + 2e^- rightarrow Cu(s) and Zn(s) rightarrow Zn^2+ (aq) + 2e^- The net reaction is Cu^2+ (aq) + Zn(s) rightarrow Cu(s) + Zn^2+ (aq) Use the given standard reduction potentials in your calculation as appropriate. Express your answer numerically to three significant figures.
Expert Answer
First, relate Eºcell
dG = -RT*ln(Keq)
dG = -n*F*Eºcell
therefore
n*F*Eºcell = RT*ln(Keq)
substitute data
Eºcell = Ered – Eox = 0.337 – – 0.763 = 1.1 V
n*F*Eºcell = RT*ln(Keq)
2*96500*1.1 = 8.314*298*ln(Keq)
lnKeq = 2*96500*1.1/(8.314*298)
Keq = exp(85.6887)
Keq = 1.6373*10^37
Keq = 1.64*10^37 (to 3 sig. fig)

