Part One Part Two
Part Two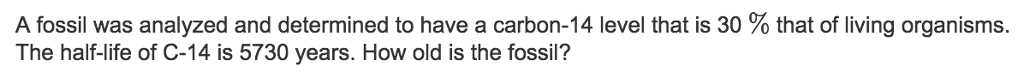
Americium-241 is used in some smoke detectors. It is an alpha emitter with a half-life of 432 years. How long will it take in years for 33.0 % of an Am-241 sample to decay?
Expert Answer
Radioactive disintegration is a first order process,
Let the initial activity of Americium-241 is a0 = 100 % and the final activity is a = 100 % – 33 % = 67 %.
The half life period, t1/2 = 432 year
For the first order reaction, k = 0.693/t1/2 = 0.693/432 = 0.0016 year-1
Applying first order kinetic equation
t = 2.303/k log (a0/a)
= 2.303/0.0016 year-1 log (100/67)
= 1439.375 year log (1.493)
= 1439.375 year X 0.17393
= 250.34 years

