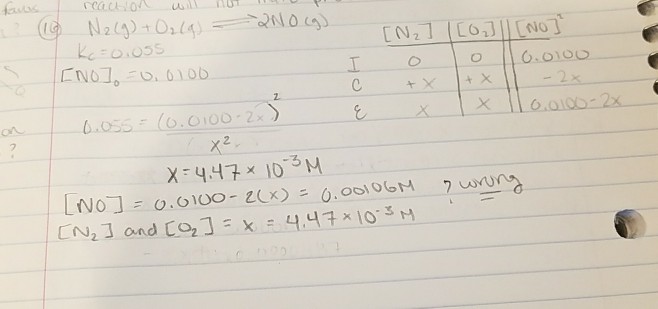The second picture is my work but it shows the answer I have is wrong.. please provide DETAILED answer since i dont see what part i did wrong
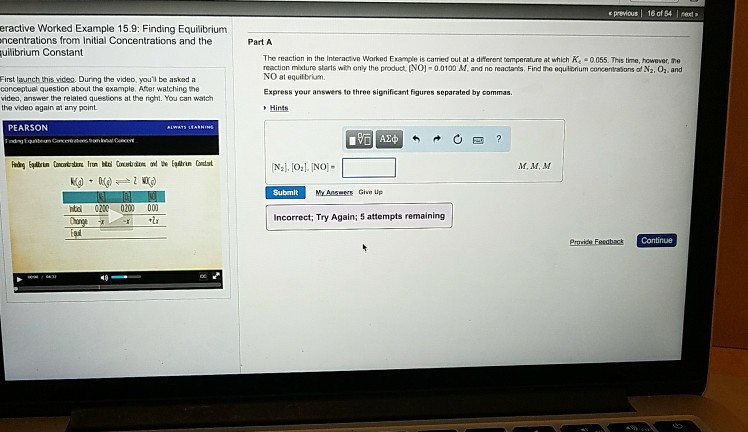
previous 16 of 54 net eractive Worked Example 15.9: Finding Equilibrium ncentrations from Initial Concentrations and the uilibrium Constant Part A The reaction in the Interactive Warked Example is carried out at a diterent temperature at which Ke 0055. This time, however, the reaction mixture starts with only the product NOI-0.01, and no reactants. Find the equtbnum concentrations of N2, 01. and NO at equilbrium. First launch this vides. During the video,you’ll be asked a conceptual question about the example. Ater watchingthe videa, answer the relaled questions at the right. You can watch the video again at any point Express your answers to three significant figures separated by commas PEARSON M. M,M Submit ntd 0200 000 000 Incorrect; Try Again; 5 attempts remaining 40
Expert Answer
Reaction taking place is:
N2 + O2 —> 2NO
initial 0 0 0.01
Eqb x x 0.01-2x
K = [NO]2/( [N2][O2] ) = (0.01-2x)2/(x2) = 0.055
Solving we get:
x = 0.0044
So,
[NO] = 0.01-2*0.0044 = 0.0012 M
[N2] = [O2] = 0.0044 M