So the response I get when I try the above answer is that the C–Br bond is replaced with a C–O bond, but it asks me where the O must come from in the EtOH. Which of the two bonds to O is more likely to break when the O in EtOH makes a new bond? Please show the correct answer.
AACC Question-answerin × + ← →。 凸 ACE epuch.uky.eduface/hornework/answer rarne.jsp?prevMode-0&cunerioNu-10&showCluck-lalse My Courses My Profile CHE 230-001 Summer 2017 Course Home Grade Book MarvinJS ch 8-elimination Maximum ellowed tries per question. Unlimited (10) Draw the major organic product o the rnartinn Fo ow this proc durn den rmn: whether the martion rnnit ons are acdにcr hasn: entry the mat nucleophilicbasic alorn, the electrophiic atorn, and Uhe leaving group, predict whether elimination or subettution will eccur, and then craw the preduct. Indicate the storcochemistry every storcoconter with single wodgod lup1. nashod (down), or wavy ta mature of up and down; orther》 bcnd. or click image to copy source H3C CH3 OH EtOH Br Br Your rasponse is incorrect (18 attempts). Yes. Ihe C Br bund is replaced willi d C O bund bul Ihe O rnus tur”el’um CIOH which urhe tu bunds lu O s more likely to break when the O in EOH rnakes a new bond? Submlt Response Question ListAssignments List 4:37 PM B1/2017
Expert Answer
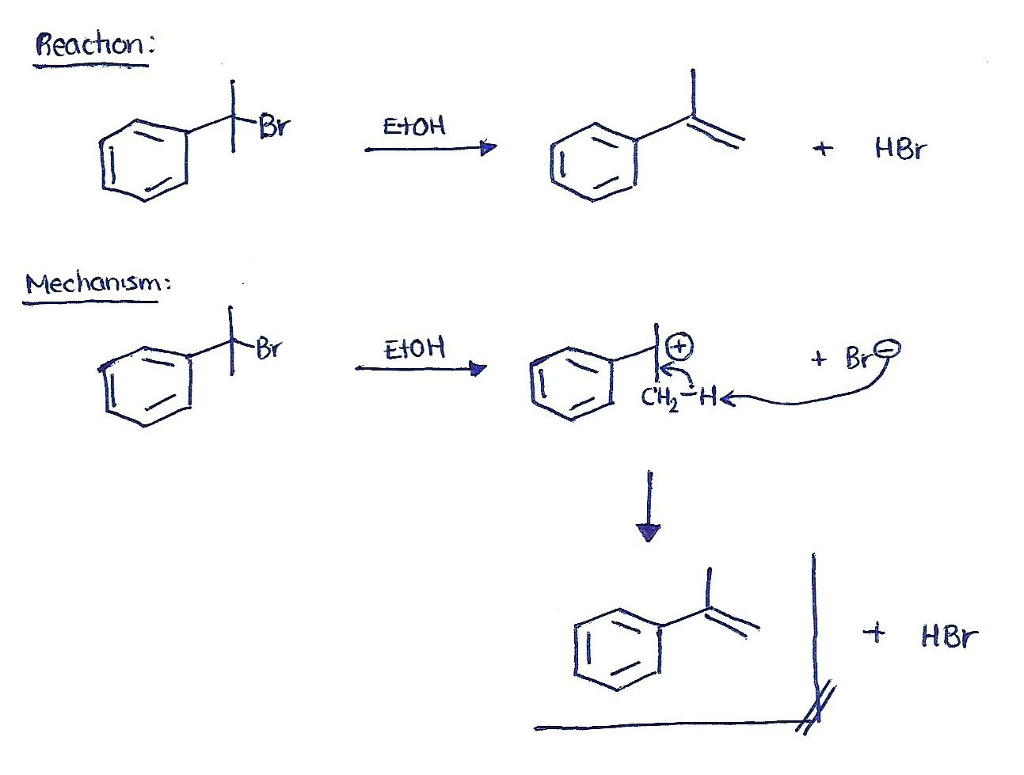
This is an elimination reaction and the product we expect is an alkene, in this case the only alkene that can be formed is that with a double bond between the benzyl carbon (the carbon that is directly bonded to the benzene group) and any of the two methyl groups.
The reaction will most likely follow the E1 reaction mechanism because the tertiary phenyl-conjugate carbocation that is formed in the first step is very stable. On a second step, the Br– that is released grabs a -H from any of the -CH3 groups and the double bond is formed: