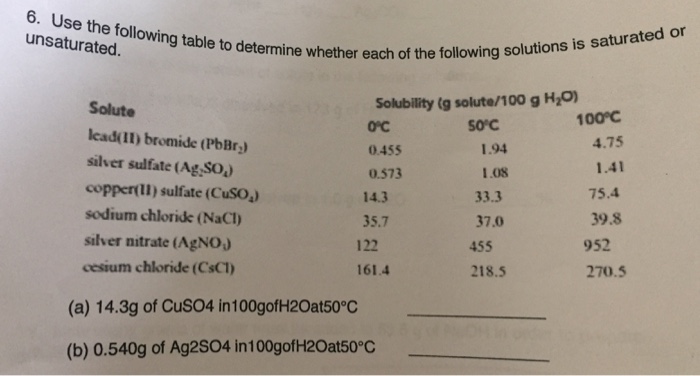
Use the following table to determine whether each of the following solution is saturated or unsaturated. (a) 14.3g of CuSO4 in 100 g of H_2O at 50 degree C _____ (b) 0.540g of Ag2SO4 in100 g of H_2O at 50 degree C _____
Expert Answer
Answer
a)
basis is 100 g of water, so we can compare directly
for CuSO4, in the data:
33.3 g of CuSO4 are soluble at 50 ºC
we have only 14.3 g of CuSO4, therefore, this is under the saturation point
choose unsaturated solution
b)
similar, we have the same basis so
saturation of Ag2SO4 = 1.08 g
we have only 0.54 g
therefore,
this is under the saturation point
choose unsaturated solution

