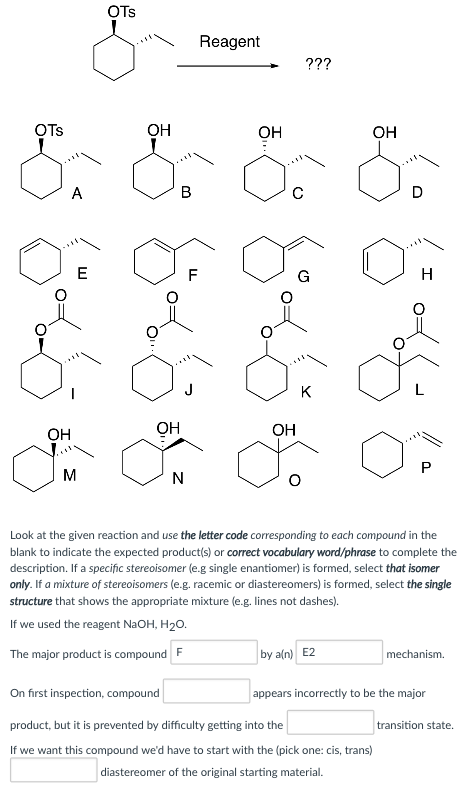

OTs Reagent OTs OH OH OH OH OH OH Look at the given reaction and use the letter code corresponding to each compound in the blank to indicate the expected products) or correct vocabulary word/phrase to complete the description. If a specific stereoisomer (e.g single enantiomer) is formed, select that isomer only. If a mixture of stereoisomers (e.g. racemic or diastereomers) is formed, select the single structure that shows the appropriate mixture (e.g. lines not dashes). If we used the reagent NaOH, H20. The major product is compoundF by a(n) E2 rs incorrectly to be the major On first inspection, compound product, but it is prevented by difficulty getting into the If we want this compound we’d have to start with the (pick one: cis, trans) appea transition state. diastereomer of the original starting material.
Expert Answer
If we use NaOH, H2O we get compound F as major product by an E2 mechanism. In the first site it appears to form compound C but get cancelled by difficulty in getting to tetrahedral transition state. To get this compound we would start with cis diastereomer of starting material.
If we convert the reagent to pure H2O. The primary products of the reaction are M and N, and F which are formed by Sn1 nd E1 mechanism. These products are formed due to an extra carbocation step in the mechanism. If this extra steps did not occur we would see compounds C and F as products instead.
If we convert the reagent first to a) CH3COONa, DMSO and then b) NaOH, H2O: Initially we will form J after the first step by an Sn2 mechanism. Then we’ll form the final product B by a Sn2 reaction.

