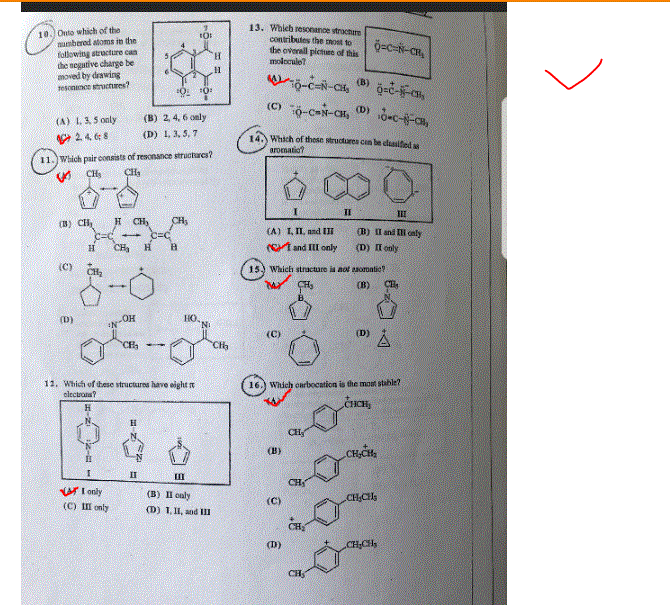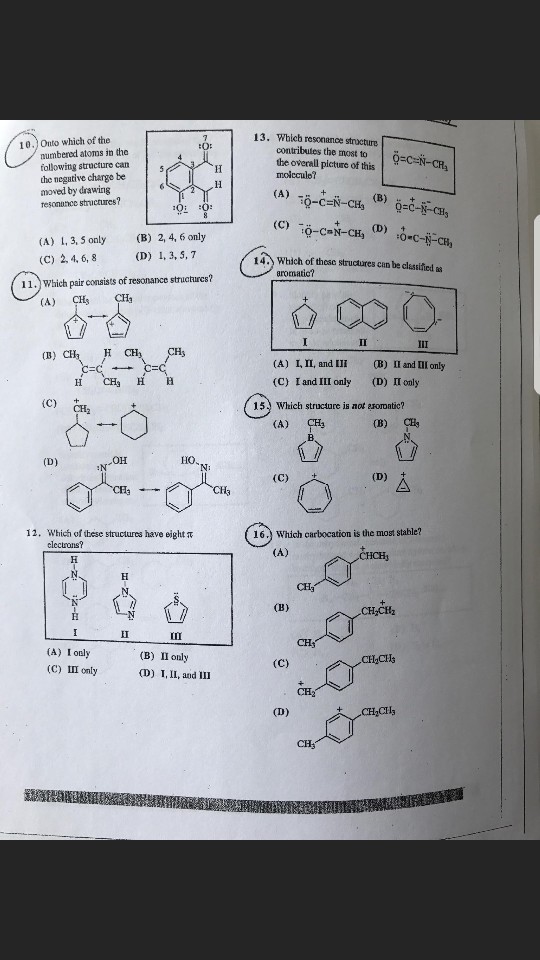
Onto which of the numbered atoms in the following structure can the negative charge be moved by drawing resonance structures? (A) 1, 3, 5 only (B) 2, 4, 6 only (C) 2, 4, 6, 8 (D) 1, 3, 5, 7 Which pair consists of resonance structures? Which of these structure have eight pi electrons? (A) I only (B) II only (C) III only (D) I, II and III Which resonance structure contributes the most to the overall picture of this molecule? Which of these structures can be classified as aromatic? (A) I, II and III (B) II and III only (C) I and II only (D) II only which structures is not aromatic? Which carbocation is the most stable?
Expert Answer
Answer