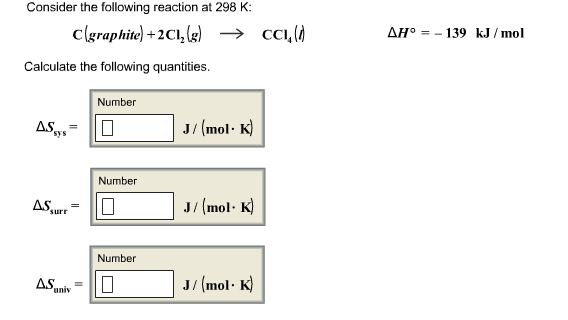
Consider the following reaction at 298 K: C(graphite) + 2Cl_2(g) rightarrow CCl_4(l) Delta H degree = -139 kJ/mol Calculate the following quantities. Delta S_sys = Delta S_surr = Delta S_univ =
Expert Answer
Answer
a)
dSystem = Sproducts – Sreactants
dSystem = s-CCl4 – (S-C + 2*S-Cl2)
dssystem = 214.4 – (5.7 + 2*223.1)
dSsystem = -237.5 J/K-mol
b)
dSsurroundings
Assume:
dSsurroundings = dQsurr/Tsurr
dQsurr = Qsurr
Qsurr = -Qsystem
Qsystem = Hrxn
Qsurr = -(-HRxn) = -(-139) = 139 kJ/mol = 139000 J/mol
dSsurroundings = 139000 J / (298K) = 466.442 J/mol-K
c)
dSsuniverse = dSsystem + dSsurroundings
dSsuniverse = -237.5 + 466.442
dSuniverse = 228.942 J/Kmol

